OPEN LETTER TO THE UK GOVERNMENT
ZDENKO KOS MSc MEc BScEcon (Hons) MBA • Oct 21, 2024
TRANSFORMATIONAL CANCER TREATMENT, Sir CHRIS HOY & UK Gov Approval

Mrs Rachel Reeves MP, Chancellor of Exchequer
Mr Jonathan Reynolds MP, Business Secretary
Mr Wes Streeting MP, Health Secretary
Mrs Lisa Nandy MP, Sport Secretary
Date: 21st October 2024
Dear Government officials
Re: Open Letter to the Government of United Kingdom
Transformational cancer treatment, Sir Chris Hoy and British Government approval
Since the announcement of Sir Chris Hoy’s terminal cancer, I have received in excess of 200 requests to help with my (our) medication/treatment. I recognise that Sir Chris, the six times Olympic Gold Winner, and his wife Sarra are in nearly every Brits heart; but all I can currently do is switch my phone off and pretend that I am not available.
You, as the government, have not been bothered to answer my and my solicitor’s recent letters. Therefore, I am currently unable to help Sir Chris Hoy and his wife Sarra, as well as thousands and thousands of cancer patients in the United Kingdom, as my hands are tied.
My (our) medications have been approved by the European Medical Association (EMA) but not by the British MHRA, which is the fault of Mr Boris Johnson all the subsequent Conservative Prime Ministers thereafter. The rest of the history you know.
I will not now be registering my medications for four (4) most common cancers in the United Kingdom as well not for the fifth - Prostate Cancer, because of all the bureaucratic issues and other issues which I have pointed out in my previous correspondence with Rachel Reeve, Jonathon Reynolds, Wes Streeting, Lisa Nandy and others.
We are in position to help the Hoys. However, to progress this our German pharmaceutical partner would need to co-ordinate everything and prepare all the necessary tablets/ cocktails/ infusions/ injections for administration either in a Swiss, German or Italian hospital.
My tablets/ cocktails/ infusions/ injections needs to be prepared, as you are already more than aware, based on the specific DNA - or for a group of similar DNAs - to have the proper effect on the patient(s).
So, as well as their consent, they would need to provide their DNA, weight, height, complete diagnose and dob data, etc, …
At the end of the day, for me the patients are always first and I would like to help at any time and any occasions whenever possible.
This, of course, include Sir Chris and his wife Sarra. As I stated, we can provide treatment in Germany or Switzerland or Italy, but not in the United Kingdom.
My children and I went through eleven distressing years supporting my wife’s battle with cancer. We realised she was being used as a guinea pig; on occasions she was given 17 different medications daily, including four which shouldn’t be used at the same time.
When I finally met with her GP, I was told that the medications were prescribed by three hospital consultants; each had prescribed different drugs.
When I made a written complaint, I didn’t receive a single answer from the hospital or from the Health Secretary.
My wife passed away in 2019 aged 46, after we were together for 28 years. I still can’t comprehend why she had to die so young. We started this research programme in 2011; it is a great regret to me that we didn’t start earlier as she might still be alive, or her life could have been prolonged.
As it stands, we now have the top medication for the five main cancers – brain, breast, colon/rectum, lung as well for the fifth - prostate cancer.
My wife’s death, my own cancers, the death of some of those close to me, and the death of a few doctors I knew – all from cancer - sparked me to go on this long journey.
I am willing to help anyone, anywhere as I know the pain and suffering endured by patients and their families.
However, as I said, in the United Kingdom my hands are tied because your Government are not interested.
Consequently, if the medications are not (pre)registered in the United Kingdom there can’t be any hospital trials, and they can’t then be used more generally in the UK.
As far as Sir Chris Hoy and his wife Sarra are concerned, they would need to join hospital trials in either Germany, Italy or Switzerland.
Based on their personal data, my (our) laboratory in Switzerland will suggest the type and strength of medication, together with the best treatment protocol.
Our German pharmaceutical partner, who has been manufacturing the medication for us for last 5 years, normally then requires 5-7 days in extreme cases they can prepare in the same day. For reference, it is this partner who wants to buy the European licence and all the patents from me.
You are aware that my medications are prepared, in layman’s terms, on the basis of repairing errors in the cellular DNA; proteins like FANC1 and FANCD2 are used along with another nine (9) which are not currently recognised and even less used in whichever combination with whatever cancer medication in the United Kingdom. With Sir Chris Hoy along with proteins there might be also use of seven (7) different hormones. Not seeing and having all the data of Sir Hoy illness I can only speculate what I will never ever when medications or treatments are concerned.
Treatment is a complex process and procedure where there is no place for cutting the corners or room for improvisation.
I have personally tested all my medications myself. In 1998 I was diagnosed with High Grade Non-Hodgkin’s Lymphoma Cancer; St Thomas’ hospital gave me 3-4 months to live. I have subsequently had another 4 cancers. By going through standard treatment along with my medications, I have tested double combined treatment to the limits.
I have also tested on myself various combinations of chemotherapy/my medications and radiotherapy/my medications.
To date, there are literally no side effects of any kind in respect of my (our) medications.
We have been working with doctors in Australia, United States, Germany, Italy, Switzerland, France and also in the United Kingdom. Our goal is, and always was, and always will be to serve patients and their families with proper medication and treatment for treating cancers and to go further and further with our research.
I would like to help Sir Chris Hoy and his family; I would also like to help the many thousands and thousands of individuals in the UK suffering with cancer. To do this, the Government needs to respond to our proposal before I give up and sell the patent to our current German Pharma partner.
Yours faithfully
Zdenko Kos MSc MEc BScEcon(Hons) MBA
https://thekosfoundation.online/prostate-cancer-standard-treatment
https://thekosfoundation.online/prostate-cancer--darexanatan
OUR FIGURES ON PROSTATE CANCER
Prostate Cancer Data
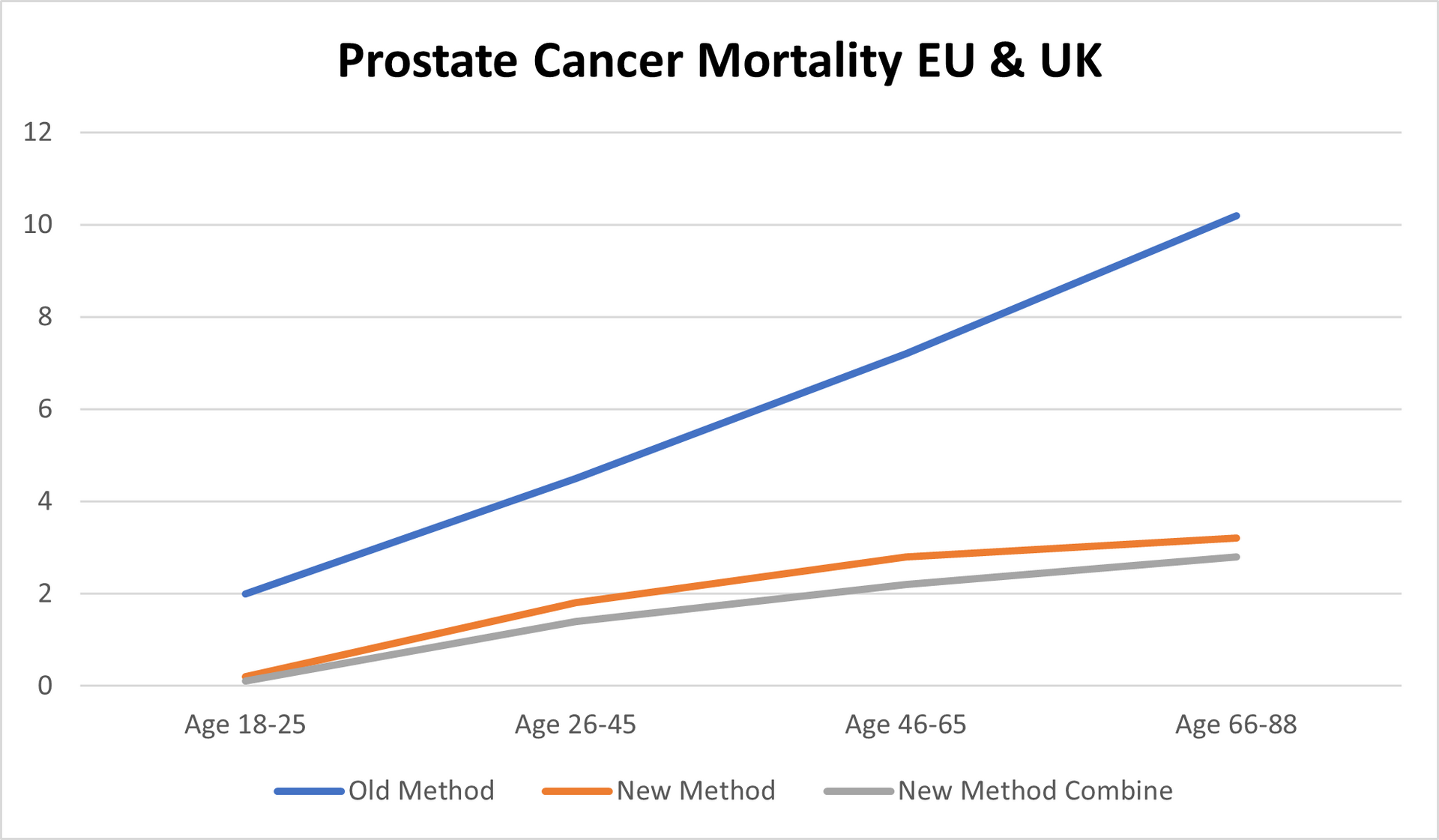
Mortality in EU 2018/23: 37,922; Mortality in UK 2018/23: 19,885 (standard treatments)
Result by Starting using our New medication/Treatment
(Testing period 2018-2023 all age groups):
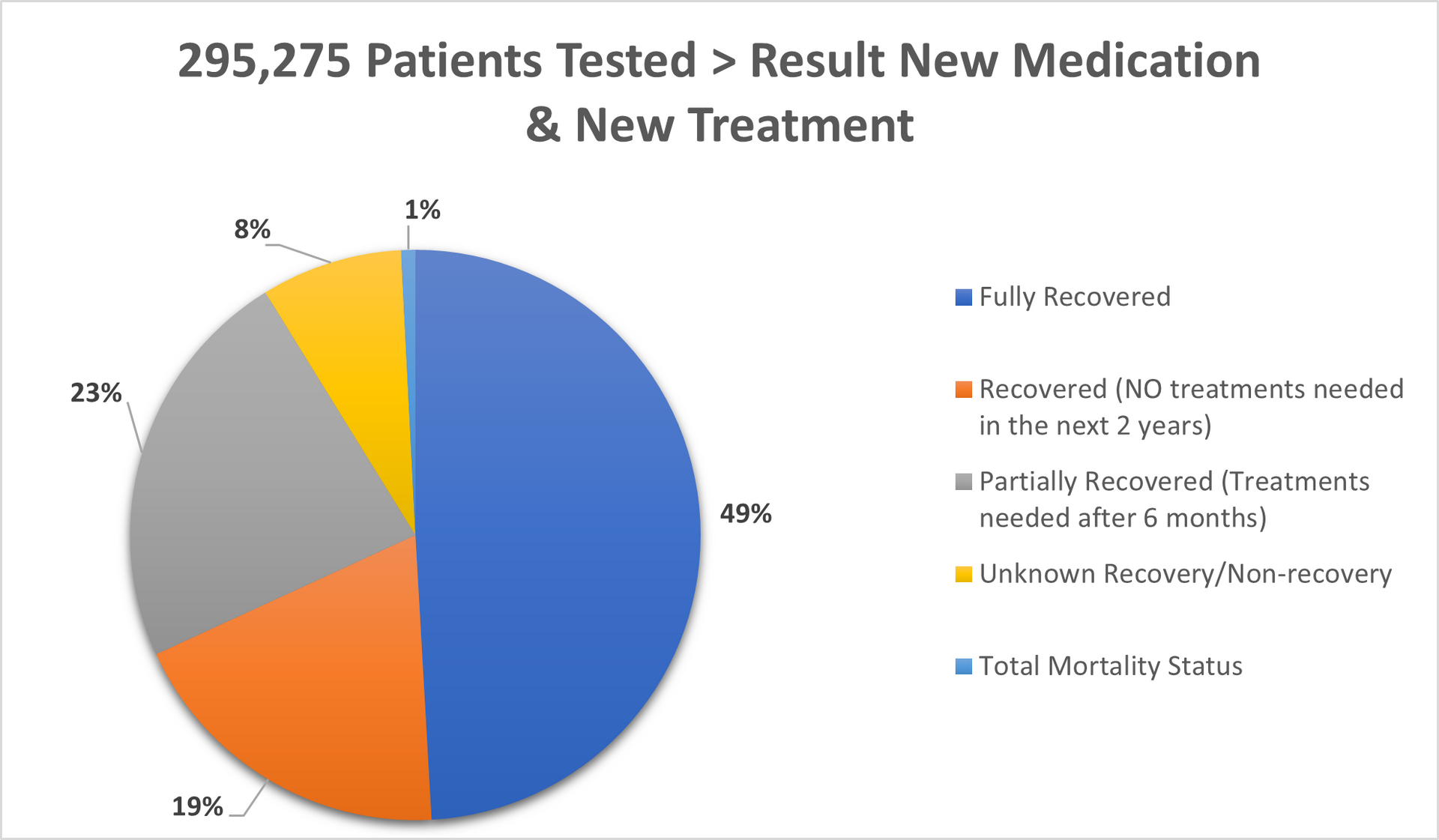
Tested patients in UK, Switzerland, Germany and Italy: 295,275
Fully recovered (all risk levels): 49%
Recovered (no further treatments needed for period of next couple of years): 19%
Partially recovered (further treatments needed as precaution after 6months passed): 23%
Unknown recovery/non-recovery: 8%
Deaths in total: less than 1% (mostly people age over 82).
REFERENCES TO THE DATA ABOVE:
Age Gap:
The youngest person was of 18 years of age
The oldest person was 88 years of age
Gender:
Female: Only 4 of 195,275 (Prostate cancer with female are very very rare)
Male: 195,271
- % of male group 18-25 was 4.2%
- % of male group 26-45 was 22.4%
- % of male group 46-65 was 30.8%
- % of male group 66-88 was 42.6%
Methods:
Old Methods meaning the standard methods used by hospital on daily basis for last minimum 5 years in EU and in the UK.
New Methods meaning our medication/treatment with which we came out after over 12 years of research and test.
New Methods combine meaning our medication/treatment combined in double parallel treatment > new medication and different way of treatment
Separate there are also results of our new medication/treatment combined with "old" methods however the final readings cannot be 100 percent accurate and cannot be as such used for whatever compares.
Mortality:
In the Europe 2018-2023 > 10.2%
In the United Kingdom 2018-2023 > 8.8%.
With our medication/treatment Europe & United Kingdom 2018-2023 > Less than 1%.
